Indiana
Our mission is to save lives by meeting the most critical needs of our communities and investing in breakthrough research to prevent and cure breast cancer.
Indiana
Our mission is to save lives by meeting the most critical needs of our communities and investing in breakthrough research to prevent and cure breast cancer.
Need Help?
Call our breast care helpline to assist with finding local screening and diagnostic facilities or clinical research trials, requesting financial assistance, or other questions or care needs.
Get Involved
Help us reach our vision of a world without breast cancer by getting involved in our local community.

Shop for the Cure
Save the date! On April 28, Rose & Remington will host a statewide fundraiser in honor of National Breast Cancer Awareness Month. This one day fundraiser will be held in store from 12-6pm EST and online with giveback code KOMEN all day on Sunday, April 28, 2024.
Learn More
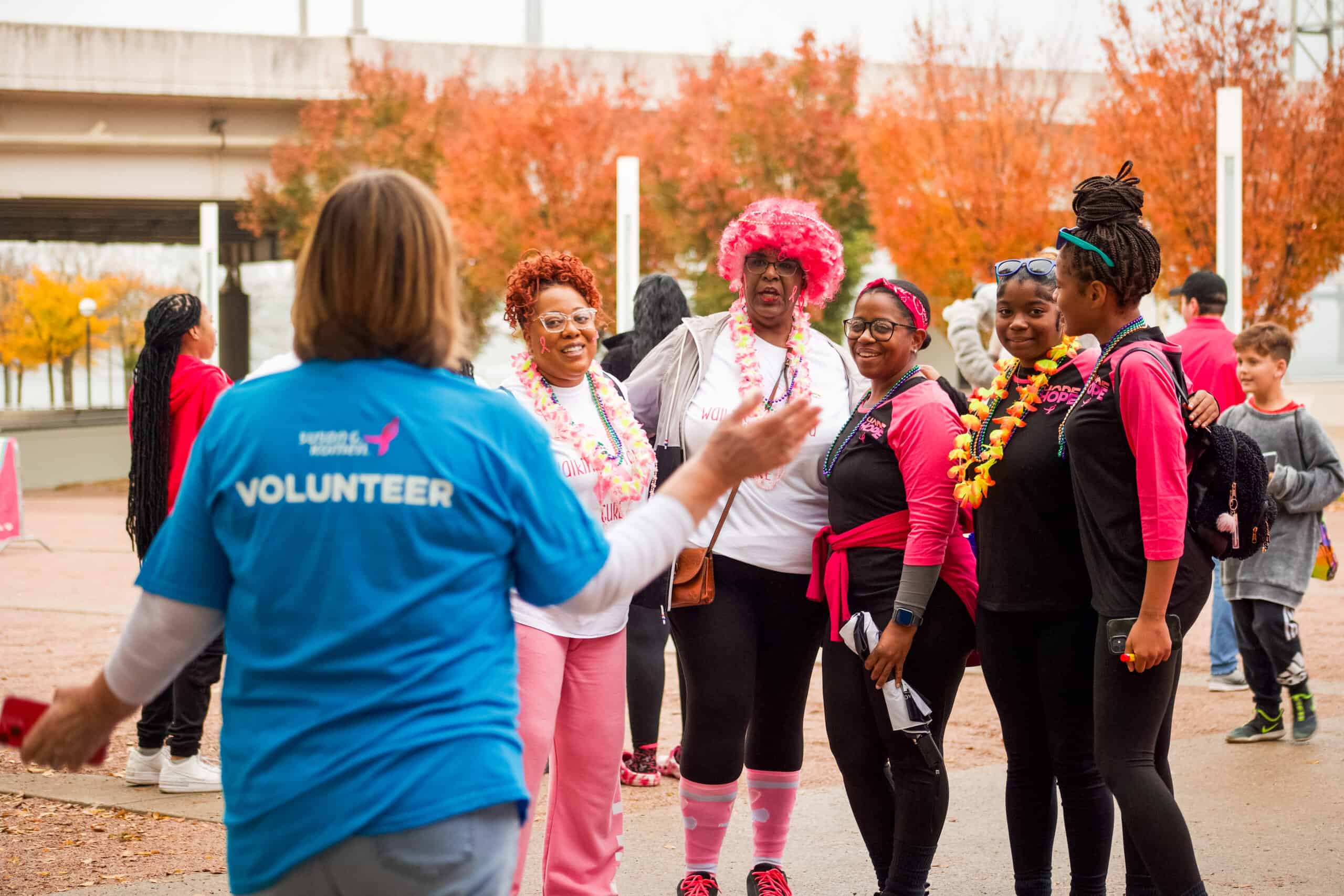
Volunteer & Make An Impact
Breast Cancer Moments
Ending breast cancer needs all of us.
From serving as a local brand ambassador and raising critical funds for our mission programs to helping breathe life into our signature fundraising events like the MORE THAN PINK Walk, Race for the Cure, and BigWig celebrations, as well as educational events like the Metastatic Breast Cancer (MBC) conference and Advocacy Summit, we have unique volunteer opportunities for everyone who wants to help us create lasting change in the lives of those facing a breast cancer diagnosis.
Find a volunteer opportunity that suits your skills and interests, and join our passionate fight against breast cancer.
General Volunteer Interest Form
Contact Us to Learn More

Engage your employees in our mission to end breast cancer forever.
When your organization partners with Komen, you’ll have access to trusted, accurate, timely breast cancer information to share with staff, and exciting team-building programs that are simple to implement. Plus, the Komen team will be ready to support, step-by-step, as you launch a corporate health and wellness campaign or employee engagement program.
Learn MoreLocal Events
Join the fight to end breast cancer by attending an event in Indiana!
Questions? Contact Us

ShareForCures
Your breast cancer information is as unique as you are. When combined with thousands of other ShareForCures members, you provide scientists with a more diverse set of data to make new discoveries, faster.
Latest News & Information
Nikki’s Story: I’m Not Going to Let Cancer Steal My Sparkle
Nikki Anderson’s cancer journey began with a uterine cancer diagnosis in her 30s, which was followed by a breast cancer diagnosis six years later. She celebrates her journey by fundraising for the Komen 3-Day with Team Sparkle, inspired by her own adage: “I’m not going to let cancer steal my sparkle.”
The post Nikki’s Story: I’m Not Going to Let Cancer Steal My Sparkle appeared first on Susan G. Komen®.
Statement on Passage of Critical Breast Health Legislation in Mississippi
JACKSON – Susan G. Komen®, the world’s leading breast cancer organization, today issued the following statement on the passage of two critical breast health bills in Mississippi. The first removes patient cost for diagnostic and supplemental imaging, a critical form of breast cancer screening for some high-risk individuals and an important step in determining the […]
The post Statement on Passage of Critical Breast Health Legislation in Mississippi appeared first on Susan G. Komen®.
Indiana
Contact Us
Susan G. Komen Indiana

State Executive Director: Jennifer Milewski
Email: jmilewski@komen.org
Phone: 463-777-6862




